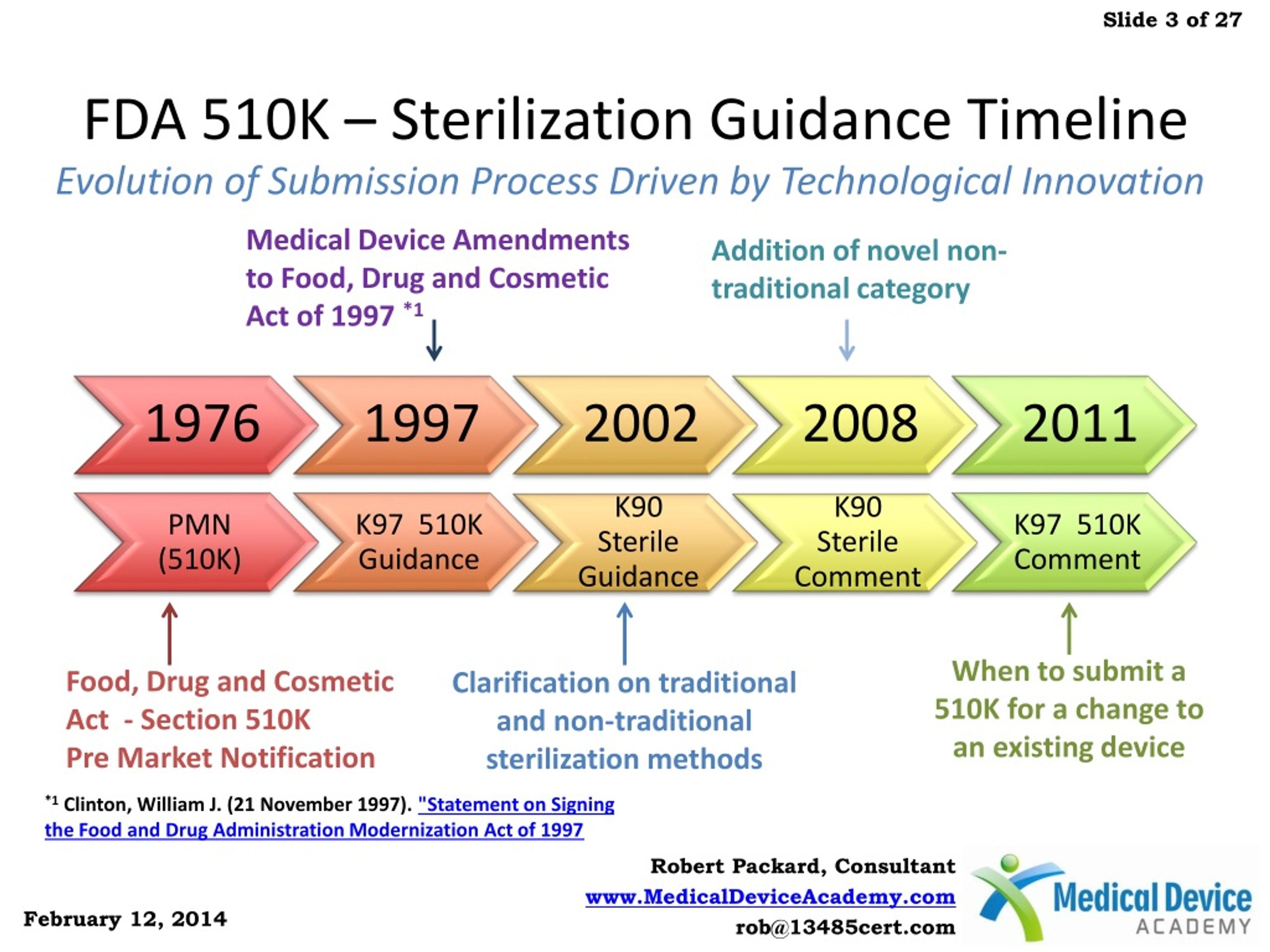
Fda Sterilization Guidance . Web recognized consensus standards: Web us fda point of consideration. This recommended practice covers steam. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. Web a list of recognized sterilization standards appears at fda's center for devices and radiological health.
from www.slideserve.com
Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. This recommended practice covers steam. Web recognized consensus standards: Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web us fda point of consideration. Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for.
PPT Why LowTemperature? PowerPoint Presentation, free download ID
Fda Sterilization Guidance Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. Web us fda point of consideration. Web recognized consensus standards: Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. This recommended practice covers steam. Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for.
From www.clikray.com
Sterilization Guidelines — ClikRay Fda Sterilization Guidance Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web recognized consensus standards: Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. Web the guidance on the content. Fda Sterilization Guidance.
From array.aami.org
510(k)s for Sterile Devices FDA Releases 2024 Final Guidance AAMI News Fda Sterilization Guidance Web recognized consensus standards: This recommended practice covers steam. Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web us fda point of consideration. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web the guidance on the content of sterile drug applications entitled guideline for the submission. Fda Sterilization Guidance.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Fda Sterilization Guidance Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web us fda point of consideration. This recommended practice covers steam. Web medical. Fda Sterilization Guidance.
From www.researchgate.net
(PDF) FDA Guide To Aseptic Processing Fda Sterilization Guidance Web us fda point of consideration. Web recognized consensus standards: Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. This. Fda Sterilization Guidance.
From www.jointcommission.org
Symbol table for device labeling Fda Sterilization Guidance Web this guidance document updates and clarifies the information regarding sterilization processes that we. This recommended practice covers steam. Web recognized consensus standards: Web us fda point of consideration. Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. Web a list of recognized sterilization standards appears at fda's center for devices. Fda Sterilization Guidance.
From www.academia.edu
(PDF) PDA's response to FDA's "Guideline on sterile drug products Fda Sterilization Guidance Web us fda point of consideration. Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. This. Fda Sterilization Guidance.
From www.slideserve.com
PPT Why LowTemperature? PowerPoint Presentation, free download ID Fda Sterilization Guidance This recommended practice covers steam. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. Web us fda point of consideration. Web recognized consensus standards: Web a list of recognized sterilization standards appears at fda's center for devices and. Fda Sterilization Guidance.
From angelanjohnson.com
Medical Devices Angela N Johnson Fda Sterilization Guidance Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web recognized consensus standards: Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web medical devices are sterilized in a variety. Fda Sterilization Guidance.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Fda Sterilization Guidance This recommended practice covers steam. Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. Web us fda point of consideration. Web a list of recognized sterilization standards appears at fda's center for. Fda Sterilization Guidance.
From www.exponent.com
FDA Finalizes EtO Sterilization Guidance Exponent Fda Sterilization Guidance Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. This recommended practice covers steam. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web us fda point of consideration. Web a list of recognized sterilization standards appears at fda's center for devices and radiological health.. Fda Sterilization Guidance.
From www.studocu.com
FDA guideline homework notes quality assurance Guidance for Fda Sterilization Guidance Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web recognized consensus standards: Web iso 11135:2014 specifies requirements for the development,. Fda Sterilization Guidance.
From operonstrategist.com
FDA's Updated Sterilization Guidelines for Medical Device Operon Fda Sterilization Guidance Web recognized consensus standards: Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. This recommended practice covers steam. Web this guidance document updates and clarifies the information regarding sterilization processes that we.. Fda Sterilization Guidance.
From www.medpagetoday.com
FDA Panel Urges New Label, More Guidance on Female Sterilization Device Fda Sterilization Guidance Web recognized consensus standards: This recommended practice covers steam. Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web iso. Fda Sterilization Guidance.
From www.scribd.com
FDA Guidance On Facing Sterile Manufacturing Inspections PDF Fda Sterilization Guidance Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. Web us fda point of consideration. Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. Web recognized. Fda Sterilization Guidance.
From www.i3cglobal.uk
FDA Medical Device Sterilization Guidance I3CGLOBAL (UK) Fda Sterilization Guidance Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web recognized consensus standards: Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web the guidance on the content of sterile drug. Fda Sterilization Guidance.
From fdocuments.in
FDA Sterile Product Manufacturing Guidelines [PDF Document] Fda Sterilization Guidance Web us fda point of consideration. Web recognized consensus standards: Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. Web the guidance on the content of sterile drug applications entitled guideline for the submission of documentation for. This recommended practice covers steam. Web this guidance document updates and clarifies the. Fda Sterilization Guidance.
From www.slideserve.com
PPT Disinfection and Sterilization Guidelines What You Need to Know Fda Sterilization Guidance Web us fda point of consideration. Web medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene. Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. Web recognized consensus standards: Web a list of recognized sterilization standards appears at fda's center for devices and. Fda Sterilization Guidance.
From www.pinterest.com
Guideline for Disinfection and Sterilization in Healthcare Facilities Fda Sterilization Guidance Web us fda point of consideration. Web recognized consensus standards: Web a list of recognized sterilization standards appears at fda's center for devices and radiological health. Web this guidance document updates and clarifies the information regarding sterilization processes that we. Web iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization. This recommended practice. Fda Sterilization Guidance.